Orbital diagrams orbitals electrons monahan 6.6: 3d representation of orbitals Which are the orbitals(s,p,d,f) have center of symmetry?
Non - directional orbital is: toppr.com
6.6: the shapes of atomic orbitals
Solved ?1. consider the s, p, and d atomic orbitals as
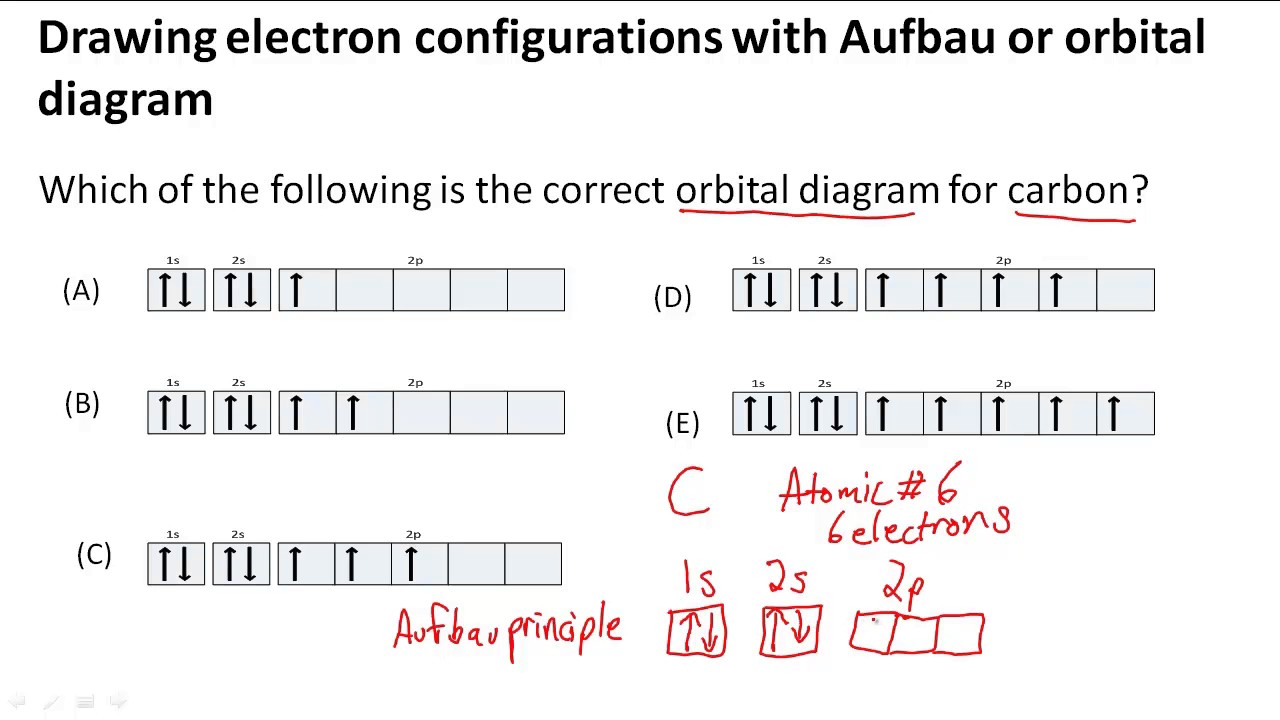
Orbitals atomic molecular between orbital difference draw 3d overlap atoms 1s following axes 2ps which molecule solved coordinateOrbital orbitals subshell symmetry socratic Orbital boundary helpedDrawing electron configurations with aufbau/orbital diagram.
Orbitals atomic shapes chemistry chem cartesian atoms structure figure size space generalDraw the boundary surface diagram for d-orbital Orbital diagrams — overview & examplesElectron configuration orbital diagram drawing diagrams configurations atoms aufbau arrangement rules example.

Orbitals electron orbital orbitali electrons atomici chemistry quantici numeri biopills atoms atom libretexts directional toppr arrangement atomo allowed nscc chem
How do you draw s,p,d,f orbitals? .
.